Canadian-led studies suggest blood thinner can help moderately sick COVID-19 patients
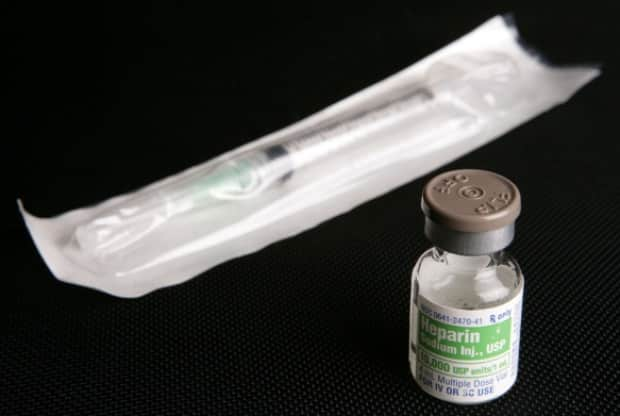
International studies led by Canadian researchers suggest full-dose blood thinners can help moderately sick COVID-19 patients and keep them off life support.
One of two studies published in the New England Journal of Medicine Wednesday shows full doses of the blood thinner heparin improved outcomes and decreased the need for life-support for moderately ill COVID-19 patients who were not in intensive care units (ICUs) and did not receive organ support at the start of the trial.
"It's encouraging that we are still finding effective treatments," said Dr. Patrick Lawler, a clinician/scientist at Toronto's Peter Munk Cardiac Centre and at the University of Toronto. He also co-led the heparin studies.
"Overall, I think that provides a lot of optimism for our ability to fight back against this terrible pandemic."
The findings could change how people with moderate COVID-19 symptoms are treated in Canadian hospitals, Lawler said.
But the second study shows that more research is still needed as to when exactly to administer heparin to patients, said Lawler and an independent expert.
The second study found the use of full-dose blood thinners on critically ill patients already requiring life-support could actually cause more harm.
"We'll do some additional studies using our data retrospectively to try to figure out if there's a more specific place where the risk-benefit tradeoff exists," Lawler said.
The trials testing out the blood thinner on both sets of patients took place in nine countries and in over 300 hospitals, including the hospitals part of Toronto's University Health Network (UHN).
The trials started in April of last year, with the critically ill trial ending in December and the other ending in January of this year.
Trial data involved 1,074 critically ill and 2,219 moderately sick patients who were given the blood thinner for up to 14 days or until they left the hospital or came off supplemental oxygen, said Lawler.
'A significant impact'
Earlier in the pandemic, it wasn't known if blood thinners would be a safe and effective treatment for COVID-19 patients in hospital.
Investigators turned to blood thinners as a treatment after seeing an increase in the number of blood clots and inflammation among COVID-19 patients worldwide.
Heparin, which is widely used in Canadian hospitals, is beneficial in the treatment of COVID-19 because it prevents clots, has an anti-inflammatory effect but could prevent COVID-19 from entering cells, said Dr. Ewan Goligher, who co-led the heparin studies.
Dr. Donald Arnold, a medical professor at Hamilton's McMaster University and a hematologist, said the findings from the two studies are significant.
"There have been a lot of interventions that have been tested, but we haven't really seen too many that have made a significant impact on clinical outcomes like this," said Arnold, who is also the medical director for the McMaster Platelet Immunology Laboratory, which has studied rare blood clots linked to COVID-19 vaccines.
"The other appealing part of that is the intervention is readily available. This is not a fancy treatment that costs a lot of money. That part was really remarkable to see."
Arnold said the two studies will spur more research as there are still some unknowns as to the exact time — or what he called "the sweet spot" — as to when heparin should be given to a moderately ill COVID-19 patient.
"I don't think we're exactly sure and we need to be careful," he said.
"But this is very compelling evidence that we now have a new treatment for the right patient that might actually benefit them in the long-run."

