More diabetes medicine recalled after testing finds too much carcinogen content
One lot of another brand of Type 2 diabetes medicine metformin, Riomet ER, has been recalled after laboratory tests revealed the drug had too much of carcinogen NDMA.
Sun Pharmaceuticals Industries, also called Sun Pharma, pulled lot No. AB06381 of Riomet, 500 mg tablets of metformin hydrochloride for extended-release oral suspension. The expiration date is 10/2021. Sun’s FDA-posted recall notice says this went to wholesalers nationwide, but didn’t state to what retailers those wholesalers sold their product.
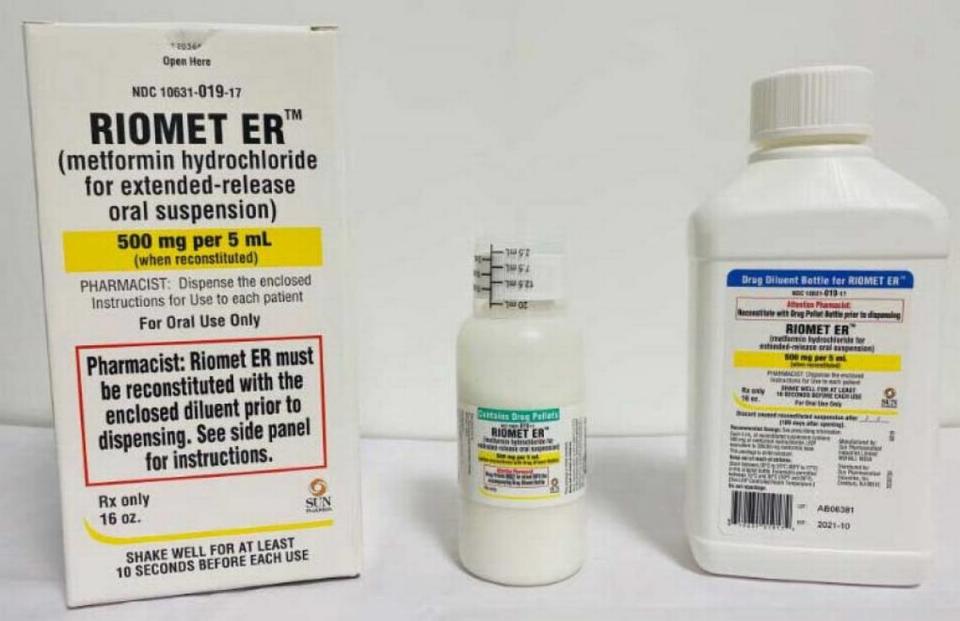
This is the ninth brand of metformin recalled for NDMA (N-Nitrosodimethylamine) since May, the full list of which can be found on the FDA’s website.
Those with questions on this recall can call Sun Pharma at 800-818-4555, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
Xanax, similar drugs carry the FDA’s most serious warning. More perils are being added
More thyroid medicines recalled for being too weak. People have reported problems


