Counterfeit Botox linked to illnesses in nine states
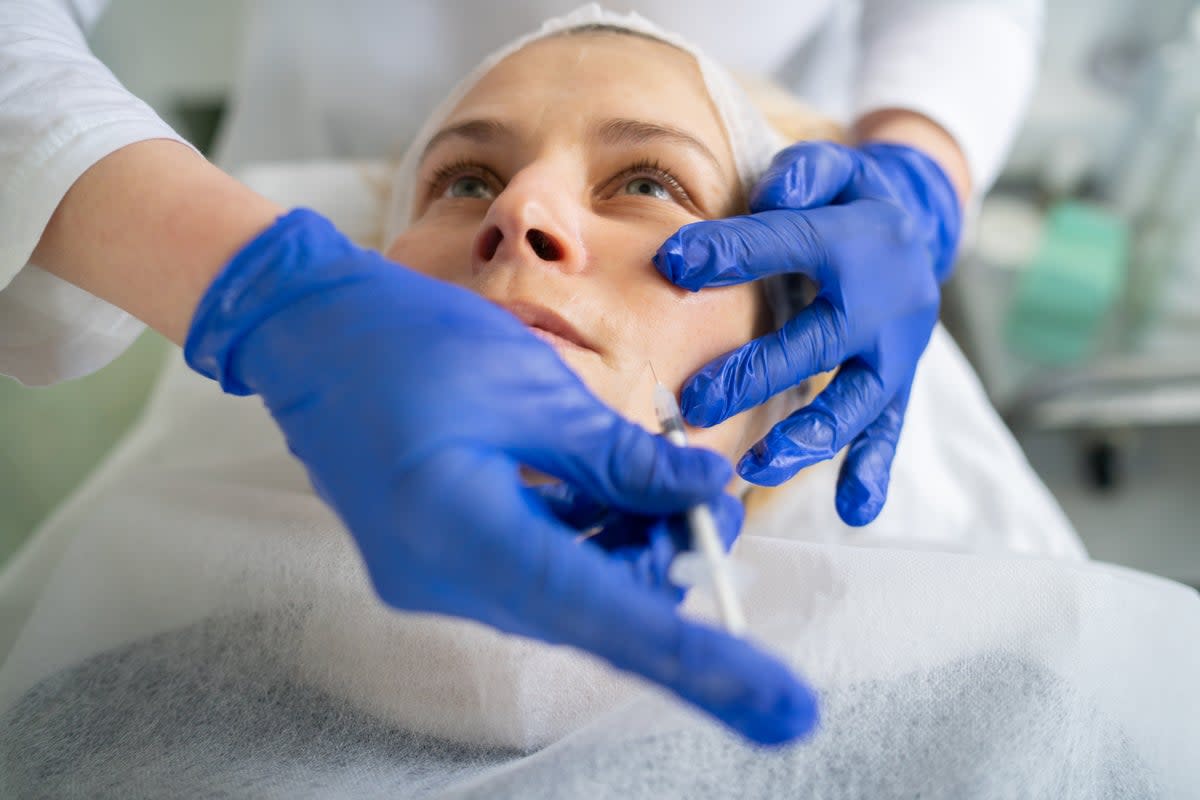
The US Food and Drug Administration found counterfeit Botox to be linked to 19 reports of illnesses across nine states.
On 16 April, the FDA released a report in which they warned healthcare professionals and consumers of the severe symptoms that are now linked to the counterfeit versions of the medication, including difficulty breathing and swallowing, dry mouth, constipation, muscle weakness, blurred vision, and loss of bladder control.
The imitation Botox (botulinum toxin) led to the development of symptoms in 19 women, nine of whom were hospitalised, four were given botulism antitoxin, and five were tested for botulism, according to the Center for Disease Control and Prevention (CDC). Botulism is a serious illness that can occur when a toxin attacks nerves in the body and can be fatal in some cases. All five women tested negative.
These women – most chose to use Botox for a cosmetic purpose – were injected by non-professionals and in “non-healthcare settings”, the CDC stated and the FDA confirmed.
“Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe,” the FDA explained.
The 19 illness reports came from Florida, Illinois, Colorado, Nebraska, Kentucky, New Jersey, New York, Tennessee, and Washington.
Counterfeit Botox shares similarities to the FDA-approved Botox manufactured by AbbVie. However, Botox made by AbbVie displays their product description on the outside of the container. The description will either be: “BOTOX® COSMETIC / onabotulinumtoxinA/for Injection” or “OnabotulinumtoxinA/BOTOX®/for injection”. The manufacturer label will read: “Allergan Aesthetics/An AbbVie Company” or “abbvie”. All FDA-approved Botox will have the active ingredient, “OnabotulinumtoxinA,” clearly typed on the outer carton and vial too.
“Currently, there is no indication that the reported events were linked to AbbVie’s FDA-approved Botox, and the genuine product should be considered safe and effective for its intended and approved uses,” the FDA proclaimed.
In pictures attached to the FDA report, the counterfeit Botox packaging, outer carton and vial included, indicated that there were 150 doses inside, listed the active ingredient as “Botulinum Toxin Type A” and the lot number “C3709C3”. The language on the package was not all English.
Michelle Waltenburg, senior botulism epidemiologist for the CDC, encouraged people who are exhibiting symptoms of botulism to immediately “seek medical care”.


