How China has emerged as a front runner in race to start human trials for Covid-19 vaccine

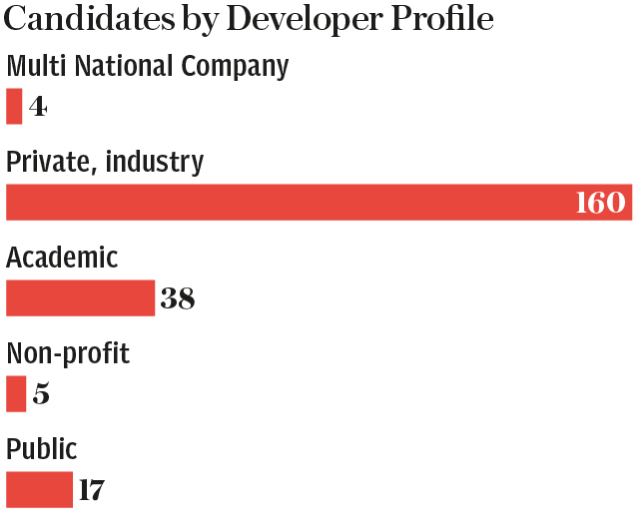
China is pulling ahead in the race to find a vaccine for the new coronavirus, with Chinese research teams accounting for 60 per cent of vaccine candidates currently in human trials.
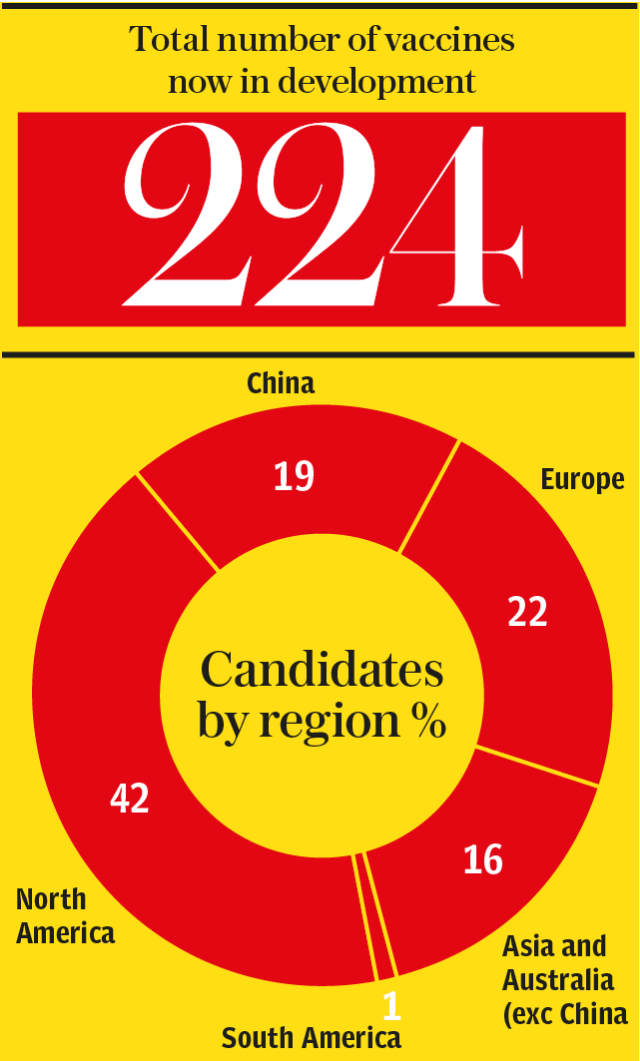
New data obtained exclusively by the Sunday Telegraph reveals that there are now 224 vaccines in development around the world - almost double the total of just a month ago.
The data, collated by Coalition for Epidemic Preparedness Innovations (Cepi), reveals that while North America has the largest number of vaccine projects underway - accounting for 49 per cent of the world’s total - China is furthest along the development track.
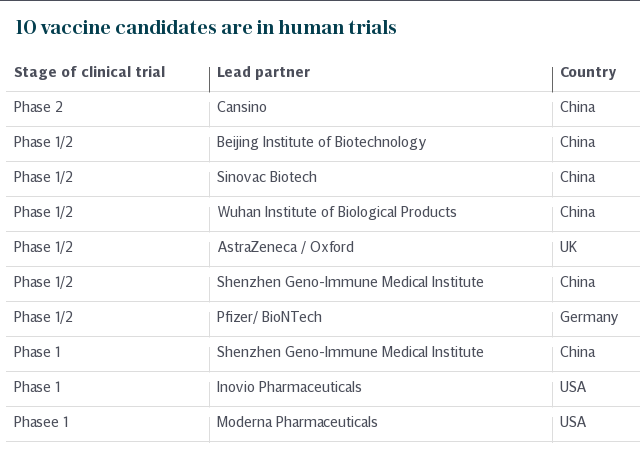
Of the 10 vaccine candidates that have progressed to human trials globally, six are Chinese and it is the only country to have a candidate now firmly into Phase II trials.
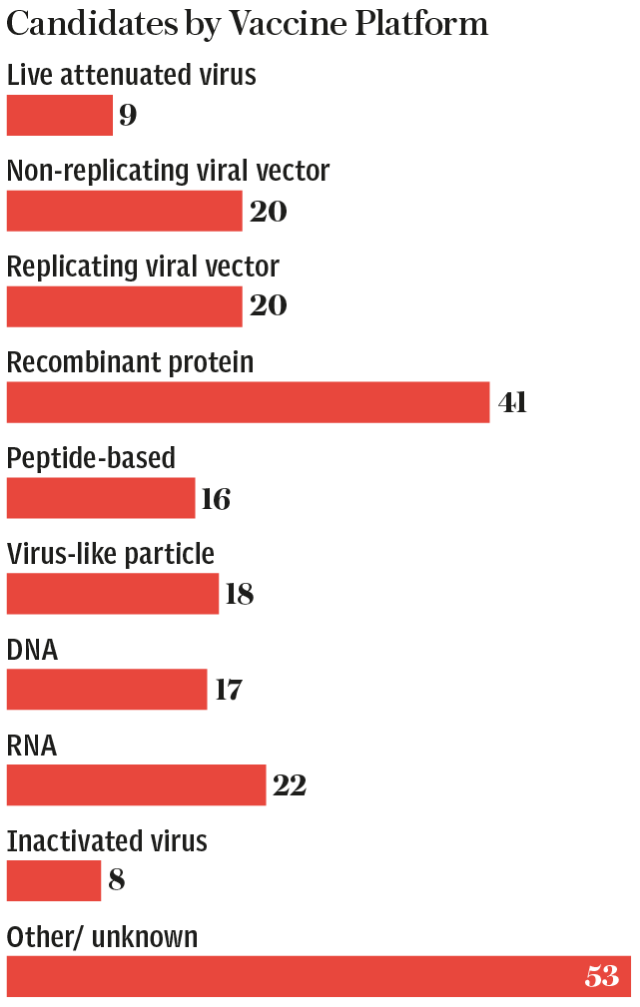
That vaccine is being pioneered by the Chinese biotech firm CanSino Biologics and the Beijing Institute of Biotechnology. It utilises a “non replicating viral-vector” design similar to the one being developed by Oxford University in England.
The results of it’s Phase I trial were reported in The Lancet on Friday. “This first-in-human trial showed that the [adenovirus five] Ad5 vectored Covid-19 vaccine was tolerable and immunogenic in healthy adults”, said the authors.
High doses of the vaccine prompted a stronger immune response but it was also associated with greater side effects. “Severe fever, fatigue, dyspnoea, muscle pain, and joint pain were reported in some of the recipients in the high dose group,” said the study.
Other leading candidates include those being developed by Sinovac Biotech in China, Pfizer and BioNTech in Germany, Moderna Pharmaceuticals in the US and Oxford University and AstraZeneca in the UK.
Dr Melanie Saville, Cepi’s director of vaccine development, said she was encouraged by the large number of vaccine candidates being developed, adding that ultimately there would need to be more than one vaccine to cover the world’s population.
She characterised the global race to find a vaccine - the biggest in history - as a “competition with the virus” and said that there continued to be good cooperation between the scientists and teams involved.
“We are seeing a lot of sharing. Companies are publishing data as it becomes available,” she said.
At the World Health Assembly earlier this week, most countries, including China, backed a resolution calling for the equitable distribution of any successful vaccines globally.
But the US disassociated itself from the move. It objected to references in the so-called "TRIPS" agreement, which allow for the compulsory sharing of intellectual property relating to medicines and vaccines during a health emergency.
In a statement, the US mission to the UN in Geneva said such language would stifle innovation and "send the wrong message to innovators who will be essential to the solutions the whole world needs".
This has fueled concerns over “vaccine nationalism” - where countries refuse to share innovations globally.
“Deals are already being made - for example by the US government - so their population gets priority access,” said Prof Devi Sridhar, chair of Global Public Health at Edinburgh University.
“The World Health Organization is trying to bring [people] together and some government’s are leading on this, including France and the UK.
“But all you need is one government not to cooperate… to misbehave and not play by the rules of the game, and it becomes very hard for everyone else too.”

As well as coordinating efforts to draft an equitable distribution agreement, the WHO is drafting a list of minimum standards successful vaccines should meet.
Its R&D Blueprint team, the same team which pioneered the concept of ‘Disease X’ to prepare for a pandemic, published a ‘Target Product Profile’ for vaccines in late April.
Among the characteristics specified it says that a successful vaccine should: provide protection for a minimum of six months; be at least 50 per cent effective; and prevent disease, severe disease and/or transmission of the virus.
And experts think it unlikely that an early Covid-19 vaccine will meet the “gold standard” for immunisation, where a vaccine prevents nearly all infections.
This sort of “sterilising immunity” is provided by only a handful of jabs including the double-dose measles vaccine - which prevents 99 per cent of infections.
A coronavirus immunisation is likely to be more comparable to a seasonal flu jab, which reduces the risk of infection by between 40 and 60 per cent, depending on the year.
“Very few vaccines give you 100 per cent protection from infection,” said Dr Saville. “Probably the most important piece at the moment is to prevent severe disease as a first step.”
Data published from clinical trials to date suggests that some of the vaccines may prevent critical illness, including pneumonia, but do not stop all Covid-19 infections.
For instance, while the vaccine candidate being developed by Oxford University prevented monkeys from suffering severe illness, early results found the viral load in the vaccinated primates noses was roughly the same level as in their unvaccinated counterparts.
Jonathan Ball, professor of molecular virology at the University of Nottingham, called for greater clarity on how the “efficacy” of Covid-19 vaccines would be defined moving forward.
“If you’ve got a vaccine that doesn’t work in vulnerable and elderly groups, but also doesn’t prevent people from transmitting Sars-CoV-2, you haven’t really got a vaccine,” he said. “These are challenges that everyone working on candidates will face.”
Experts are also eager to “manage public expectations” about the timeframe for delivering an immunisation. Most say that while it is possible “experimental” products may start to become available for health workers in the autumn, mass vaccination programmes are still at least a year away and perhaps considerably longer.
This is partly a function of the need for large scale trials to prove a product’s safety, but also because of cost and the complex logistics involved with manufacturing and distributing vaccines at scale.
“It usually takes years or decades to develop a vaccine, so really huge effort is being put into rapid development,” said Dr Saville.
“It’s too early to say that there’s one particular candidate that we’re excited about, but we really need to focus on three key areas of speed, scale and access when investing in the most promising,” she added.
Protect yourself and your family by learning more about Global Health Security


