CDC director overrules advisory panel, paving way for wider use of COVID-19 vaccine boosters

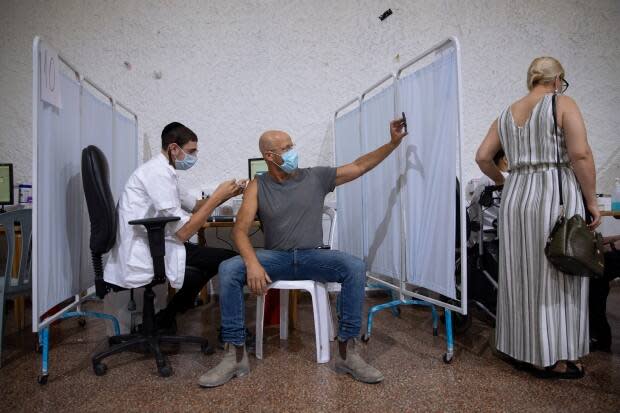
The U.S. Centers for Disease Control and Prevention (CDC) on Friday backed a booster shot of the Pfizer-BioNTech COVID-19 vaccine for Americans aged 65 and older, some adults with underlying medical conditions, and some adults in high-risk working and institutional settings.
The move comes after an advisory panel to the agency on Thursday did not recommend that people in high-risk jobs, such as health-care workers, teachers and risky living conditions should get boosters. The panel had recommended boosters for elderly and some people with underlying medical conditions.
CDC director Rochelle Walensky said her agency had to make recommendations based on complex, often imperfect data.
"In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good," she said in a statement.
"I believe we can best serve the nation's public health needs by providing booster doses for the elderly, those in long-term care facilities, people with underlying medical conditions, and for adults at high risk of disease from occupational and institutional exposures to COVID-19. This aligns with the FDAs booster authorization and makes these groups eligible for a booster shot."
The CDC recommendation follows U.S. Food and Drug Administration authorization and clears the way for a booster rollout to begin as soon as this week for millions of people who had their second dose of the Pfizer shot at least six months ago.
President Joe Biden praised the decision at a news conference Friday morning and said he would be receiving an additional dose in the near future.
Biden assured the tens of millions of Americans who received Moderna and Johnson & Johnson doses that they "still have a high degree of protection," and that the scientific community was monitoring the data on when booster shots for those vaccines might be necessary.
Decision affects those jabbed 6 or more months ago
The CDC said that people 65 years and older should get a booster. Beyond older Americans, the CDC also recommended the shots for all adults over 50 with underlying conditions.
It said that, based on individual benefits and risks, 18- to 49-year-olds with underlying medical conditions may get a booster, and people 18 to 64 at increased risk of exposure and transmission due to occupational or institutional setting may get a shot.
The recommendations only cover people who received their second Pfizer-BioNTech shot at least six months earlier. The CDC said that group is currently about 26 million people, including 13 million age 65 or older.
The CDC's Advisory Committee on Immunization Practices on Thursday gave the thumbs down to additional doses for groups including health-care workers, teachers and residents of homeless shelters and prisons.
Panel member Lynn Bahta, who works with the Minnesota Department of Health, voted against that measure. She said the data does not support boosters in that group yet.
"The science shows that we have a really effective vaccine," she said.
The committee had said it could revisit the guidance later.
Last month, U.S. President Joe Biden and eight top health officials said they hoped to start a broad booster shot program this week, saying that emerging data showed immunity wanes over time.
Vaccine expert Dr. Paul Offit said he believed the CDC advisers were worried that recommending boosters based on employment would allow overly broad use, especially in younger people for whom the health benefits of a booster shot are still unclear.
"That was a hole that you could drive a truck through, that essentially what we were doing was basically what the [Biden] administration initially asked — to just have a vaccine for the general population, because obviously the pharmacists aren't going to figure out whether you're working in a grocery store or hospital," he said.
Vaccination campaign slowing
More than 180 million people in the United States are fully vaccinated, or about 64 per cent of the eligible population. The rate has seen the U.S. passed by several countries despite getting an earlier start to its inoculation campaign.
Biden in his speech blasted elected officials "actively working to undermine" that campaign by spreading misinformation about the safety of vaccines.
WATCH | WHO adviser raises concerns about COVID-19 vaccine booster shots:
Some countries, including Israel and the United Kingdom, have already begun COVID-19 booster campaigns, despite condemnation of the practice from the World Health Organization. WHO cites global vaccine inequity, with many countries below 10 per cent on first doses.
Canada's national advisory body on vaccines currently recommends giving third doses of COVID-19 vaccines to certain immunocompromised individuals, but hasn't decided whether to provide additional shots to the broader population.
The recommendations from the National Advisory Committee on Immunization (NACI) stipulate that moderately to severely immunocompromised Canadians should be vaccinated with a primary series of three doses of an authorized mRNA-based vaccine, which includes those from Pfizer-BioNTech and Moderna.

