Why AstraZeneca vaccine approval in Canada may open more doors
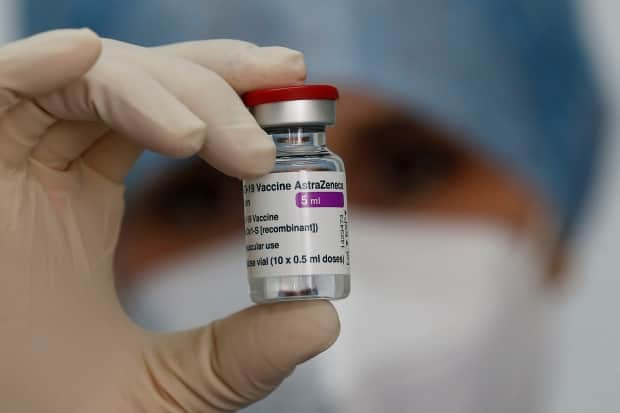
Health Canada's approval of the Oxford-AstraZeneca and the Serum Institute of India's version to prevent COVID-19 in adults follows similar green lights from regulators in the United Kingdom, Europe Union, Mexico and India.
The Oxford-AstraZeneca vaccine, called ChAdOx1, was approved for use in Canada on Friday following clinical trials in the United Kingdom and Brazil that showed a 62.1 per cent efficacy in reducing symptomatic cases of COVID-19 cases among those given the vaccine. Experts have said any vaccine with an efficacy rate of over 50 per cent could help stop outbreaks.
Dr. Supriya Sharma, Health Canada's chief medical adviser, said the key number across all of the clinical trials for those who received AstraZeneca's product was zero — no deaths, no hospitalizations for serious COVID-19 and no deaths because of an adverse effect of the vaccine.
"I think Canada is hungry for vaccines," Sharma said in a briefing. "We're putting more on the buffet table to be used."
Specifically, 64 of 5,258 in the vaccination group got COVID-19 with symptoms compared with people in the control group given injections (154 of 5,210 got COVID-19 with symptoms).
Dr. Susy Hota, medical director of infection prevention and control at Toronto's University Health Network, called it a positive move to have AstraZeneca's vaccines added to Canada's options.
"Even though the final efficacy of the AstraZeneca vaccine appears lower than what we have with the mRNA vaccines, it's still reasonably good," Hota said.
"What we need to be focusing on is trying to get as many people as possible vaccinated so we can prevent the harms from this."
Canada has an agreement with AstraZeneca to buy 20 million doses as well as between 1.9 million and 3.2 million doses through the global vaccine-sharing initiative known as COVAX.
WATCH | AstraZeneca vaccine overview:
Canada will also receive 2 million doses of AstraZeneca's COVID-19 vaccine manufactured by the Serum Institute of India, the government announced Friday.
Here's a look at some common questions about the vaccine, how it works, in whom and how it could be rolled out.
What's different about this shot?
The Oxford-AstraZeneca is cheaper and easier to handle than the mRNA vaccines from Pfizer-BioNTech and Moderna, which need to be stored at ultracold temperatures to protect the fragile genetic material.
AstraZeneca says its vaccine can be stored, transported and handled at normal refrigerated conditions (2 to 8 C) for at least six months. (Moderna's product can be stored at refrigeration temperatures for 30 days after thawing.)
The ease of handling could make it easier to administer AstraZeneca's vaccine in rural and remote areas of Canada and the world.
"There are definitely some advantages to having multiple vaccine candidates available to get to as many Canadians as possible," Hota said.
Sharma said while the product monograph notes that evidence for people over age 65 is limited, real-world data from countries already using AstraZeneca's vaccine suggest it is safe and effective among older age groups.
"We have real-world evidence from Scotland and the U.K. for people that have been dosed that would have been over 80 and that has shown significant drop in hospitalizations," Sharma said, based on a preprint.
Data from clinical trials is more limited compared with in real-world settings that reflect people from different age groups, medical conditions and other factors.
How does it work?
Vaccines work by training our immune system to recognize an invader.
The first two vaccines to protect against COVID-19 that were approved for use in Canada deliver RNA that encodes the spike protein on the surface of the pandemic coronavirus.
In contrast, the AstraZeneca vaccine packs the genetic information for the spike protein in the shell of a virus that causes the common cold in chimpanzees. Vaccine makers altered the adenovirus so it can't grow in humans.
Viral vector vaccines mimic viral infection more closely than some other kinds of vaccines. One disadvantage of viral vectors is that if a person has immunity toward a particular vector, the vaccine won't work as well. But people are unlikely to have been exposed to a chimpanzee adenovirus.
AstraZeneca is working on reformulating its vaccine to address more transmissible variants of coronavirus.
How and where could it be used?
Virologist Eric Arts at Western University in London, Ont., said vaccines from Oxford-AstraZeneca, Johnson & Johnson, which is also under review by Health Canada, and Russian Sputnik-V vaccines all have some similarities.
"I do like the fact that AstraZeneca has decided to continue trials, to work with the Russians on the Sputnik-V vaccine combination," said Arts, who holds the Canada Research Chair in HIV pathogenesis and viral control.
"The reason why I'm encouraged by it is I think there might be greater opportunity to administer those vaccines in low- to middle-income countries. We need that. I think our high-income countries have somewhat ignored the situation that is more significant globally."
Researchers reported on Feb. 2 in the journal Lancet that in a Phase 3 clinical trial involving about 20,000 people in Russia, the two-dose Sputnik-V vaccine was about 91 per cent effective and appears to prevent inoculated individuals from becoming severely ill with COVID-19.
WATCH | Performance of AstraZeneca's COVID-19 vaccine so far:
There were 16 COVID-19 cases in the vaccine group (0.1 per cent or 16/14,964) and 62 cases (1.3 per cent or [62/4,902) in the control group.
No serious adverse events were associated with vaccination. Most adverse events were mild, such as flu-like symptoms, pain at injection site and weakness or low energy.
Arts and other scientists acknowledged the speed and lack of transparency of the Russian vaccination program. But British scientists Ian Jones and Polly Roy wrote in an accompanying commentary that the results are clear and add another vaccine option to reduce the incidence of COVID-19.

