Why Canada is taking so long to start testing blood for COVID-19
With shortages of masks, gloves and testing kits hampering the fight against the coronavirus in Canada, there is one important weapon that remains unused — rapid blood tests that will tell within 15 minutes if someone has been exposed.
These tests cannot detect early infections because the body's immune system hasn't had time to produce antibodies against the virus. But about five to seven days after symptoms show up, they could be used to determine who has been infected and who has not — which would provide a more accurate picture of Canada's epidemic, including identifying people who were asymptomatic or had only mild infections.
The rapid blood tests are already being used in Europe, Asia, Australia and the U.S. Some academic laboratories are also developing COVID blood tests.
But so far none of those tests has been approved for use in Canada.
One company, BTNX Inc., in Markham, Ont., is shipping thousands of rapid tests to hospitals in the U.S.
Mitchell Pittaway, the company's chief financial officer, said he would rather be distributing the tests in Canada.
"The response [from Health Canada] has been a bit longer than what we would have liked to have seen," Pittaway said. "The U.S. has been much quicker."
Similar to blood glucose test
This week his company shipped 20,000 tests to U.S. hospitals. It will be sending another 200,000 to the U.S. next week.
"As a Canadian company with Canadian staff, ideally we would love to have all of this capacity blocked off for Canada, but we're not able to step back and not address needs coming from other countries."

The test kit sells for about $10 US and it uses a simple finger-prick of blood to reveal whether someone has been infected with SARS-CoV-2, the virus that causes COVID-19.
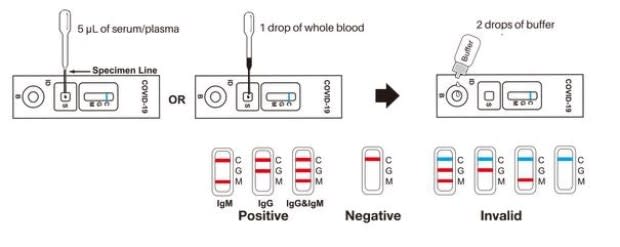
"It's similar to a blood glucose test," said Pittaway. "Put a droplet of blood in the sample well, followed by two droplets of buffer, and after 15 minutes there will be a reaction." That's if the person has been infected with COVID-19. There will be no red lines on the test paper if there are no antibodies detected in the blood.
The company reports that the test is highly sensitive and specific for the COVID-19 virus, but does not recommend it as stand-alone confirmation of an infection, and the test can be complicated if a person has been infected by milder coronavirus strains.
Still, Pittaway said the test could help reduce the current strain on the laboratory testing system by prioritizing anyone who gets a positive result on the blood test.
Health Canada reviewing
There is a risk of false positives and false negatives with any test. That's one reason Health Canada is taking a close look at the rapid blood tests before approving them for use in Canada
"The department is working with the National Microbiology Laboratory to validate testing and research, along with expert advice, so that we can have confidence in the test results," Health Canada spokesperson Geoffroy Legault-Thivierge said in an email.
In the U.S., the FDA granted expedited approval for their use by health care professionals only.
"And that's the same as we're pursuing in Canada," said Pittaway There are more than a dozen companies ready with blood tests and waiting for Health Canada to give them the go-ahead.
"Our application to Health Canada was submitted and under review," Bryan Fang, a spokesperson for Healgen Scientific said in an email. "Health Canada will release the information once it is approved."
Fang said his company received European approval back in February to supply a rapid COVID-19 test to the U.K., France and Italy.
"We are increasing our capacity every day," Fang said Wednesday. "As of today, we can make 500-600K per day."
Swab test kits prioritized
Two weeks ago, Canada's health minister signed an interim order to speed up approvals for COVID-19 tests and other medical devices. Rapid blood tests are being considered under that order
"These tests are also being accepted for review; however, the World Health Organization does not currently recommend serological tests for clinical diagnosis, and Health Canada is following this advice," said Legault-Thivierge, adding that Health Canada officials are giving the traditional PCR (polymerase chain reaction) testing kits priority under the interim order.
The laboratory-based PCR test is the only one that can detect an infection in the early stages, using a nasal swab inserted into a patient's nose. That sample is sent to a hospital or provincial lab, where specialized machines try to detect the virus's genetic material.
But a cross-Canada shortage of testing kits, machines and trained laboratory staff means many COVID-19 cases are being missed, and provincial case counts are underestimating the extent of the epidemic in Canada.
People are still shocked to learn that they might not be tested despite having symptoms or being in close contact with someone who is infected. Even in nursing homes with active COVID-19 outbreaks, Ontario is still only testing people with symptoms.
Dr. Samir Sinha, director of geriatrics at Toronto's Sinai Health Systems, told CBC Toronto's Mike Crawley that everyone in an affected long-term care facility should be tested.
"That's important so that we don't miss cases that could allow us to further spread this virus around and potentially kill more people," he said.
Across Canada, researchers are scrambling to increase PCR testing capacity. At the University of Toronto, Keith Pardee is developing a portable test system that uses different chemicals than the ones currently running short. His test is not yet approved for use, but he hopes to have patient trials completed by early next month.
At the University of Calgary, Dr. Dylan Pillai is developing another alternative to the PCR test that would also be portable and use different chemicals. He said he could be ready within two weeks to help take the load off the laboratories.
"We have a working assay already developed and we're just in the process of validating that," he said.
With new research suggesting there could be a substantial number of people infected without showing any symptoms, experts are increasingly recommending the use of population-wide rapid blood tests as an important tool in the process of returning to normal. The blood tests will reveal how many people have already been exposed, and could therefore be expected to have at least some short-term immunity to the virus.


